Alzheimer’s Biomarker Found in Newborns—What It Reveals About Brain Health
What if the key to understanding Alzheimer’s disease lies in the blood of newborns? A 2025 study led by researchers at the University of Gothenburg found that both newborns and Alzheimer’s patients share a rare biological trait: elevated levels of p-tau217, a protein long tied to neurodegenerative diseases, but now showing a surprising connection to early brain development. This discovery could redefine how we diagnose, treat, and even prevent one of the world’s fastest-growing health crises. Let’s unpack the unsettling similarity between these two seemingly unrelated groups—and what it might mean for the future of Alzheimer’s research and newborn care.
Alzheimer’s Biomarker in Newborns: A Medical Mystery with Major Implications
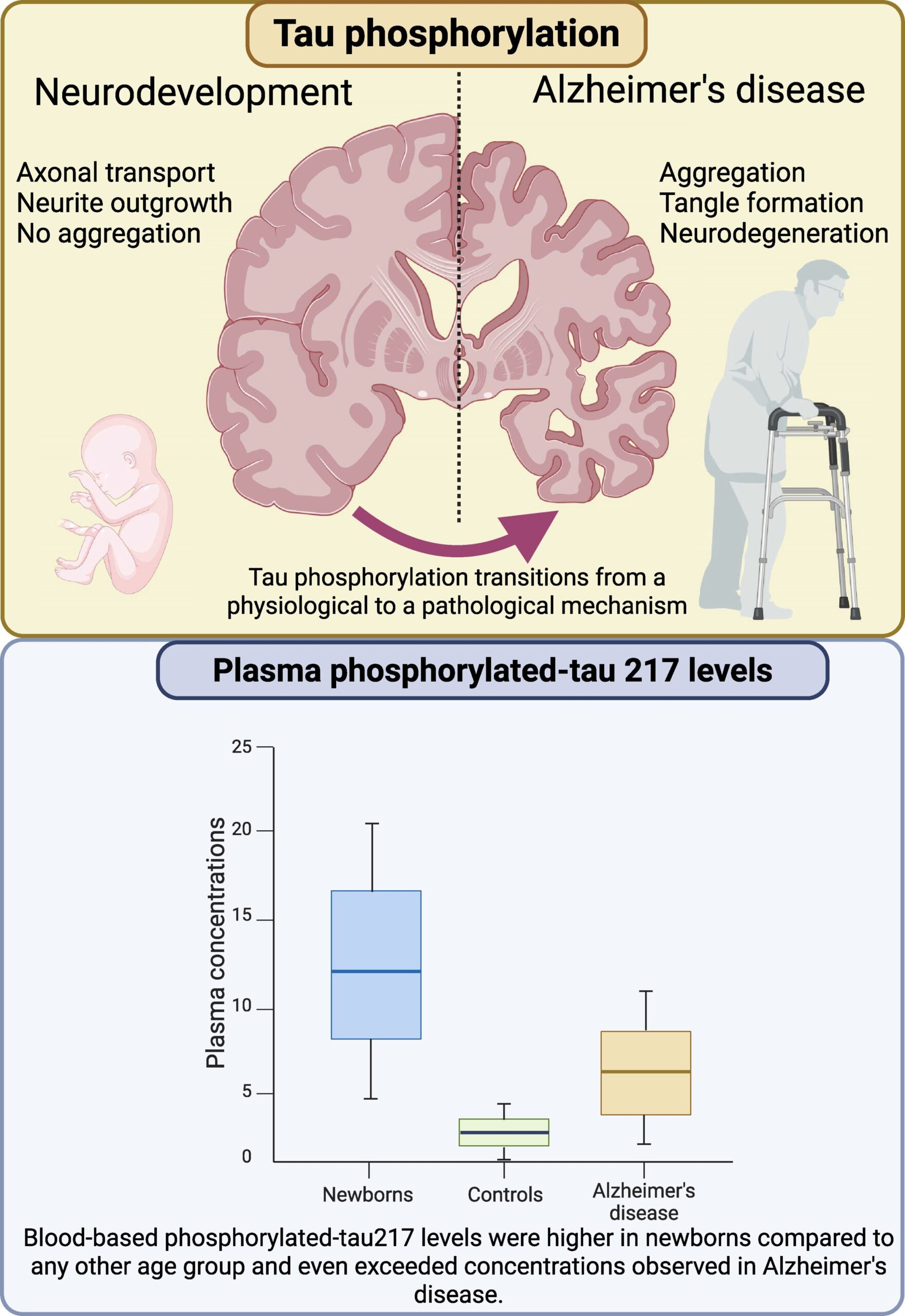
The link between **Alzheimer’s biomarker** p-tau217 and newborns is neither slight nor simple. The study, published in *Brain Communications*, revealed that healthy newborns had significantly higher concentrations of the protein compared to adults without neurodegenerative conditions. Yet, the meaning of elevated **Alzheimer’s biomarker** diverges drastically. While it signals disease progression in Alzheimer’s patients, in newborns, p-tau217 appears to support neuronal growth and synaptic plasticity crucial for cognitive development. “This is a paradigm shift,” said lead researcher Professor Kaj Blennow. “We’re used to seeing elevated **Alzheimer’s biomarker** as bad, but in babies, it might be a sign of brain resilience.”
The finding highlights the dual nature of biological signals. In the context of **Alzheimer’s biomarker** analysis, p-tau217 has served as a gold-standard indicator of amyloid build-up and neurodegeneration. However, this study challenges that narrative by suggesting the protein is far more complex: it may even play a role in the immune system’s reaction to brain development or trauma, like that seen in **neurodegenerative disease** cases. The implications are staggering. “If we can understand how newborns tolerate high p-tau217 levels, we might uncover pathways to prevent Alzheimer’s later in life,” said Blennow. The study’s focus on **Alzheimer’s biomarker** reveals a critical gap in current diagnostics, as the protein’s dual role complicates traditional interpretations.
One thing is clear: the **Alzheimer’s biomarker** connection to newborns is more than just curious. The study’s largest dataset tracked perinatal asphyxia, a severe condition causing brain cell death in full-term infants. Researchers found that **Alzheimer’s biomarker** levels correlated strongly with gestational age—earlier-born infants (before 37 weeks) had notably higher plasma **Alzheimer’s biomarker** concentrations, suggesting a link between underdeveloped brain structures and the protein’s behavior. Yet again, the findings defy expectation. While the **Alzheimer’s biomarker** is a hallmark of disease, it may also be a part of the brain’s adaptive mechanisms, a revelation that could change the game for neonatal care and age-related dementia. For parents and caregivers, the takeaway is nuanced: high **Alzheimer’s biomarker** levels in newborns are not a warning, but a potential clue.
Unraveling the Role of Tau Protein in Brain Health
Tau protein, a key player in Alzheimer’s disease, acts as a scaffold for microtubules. Its phosphorylated form, p-tau217, has become a vital marker for detecting neurodegeneration in adults. However, the University of Gothenburg study now reveals that this same protein behaves differently in newborns. Neonatal brains show **Alzheimer’s biomarker** elevations without signs of degeneration, pointing to unique protective or developmental mechanisms. “We’re seeing how the brain uses **tau protein** to build resilience in the first days of life,” said Blennow. “If only we could replicate this in aging populations, we’d be revolutionizing Alzheimer’s treatment.”
The research builds on a 2024 global review of **tau protein** dynamics, which found that 34% of Alzheimer’s patients had elevated p-tau217 levels before symptoms appeared. However, the newest data from newborns adds a twist: their bodies produce the protein in abundance. “This suggests that **tau protein** isn’t inherently harmful,” said Dr. Sofia Alvarez, a neurochemist at the University of Sussex. “It’s context-dependent, much like a fire can be a destructive force or a source of warmth for a campsite.” The challenge now is to determine how the **Alzheimer’s biomarker** functions in these two opposing roles—and how to leverage that knowledge for clinical breakthroughs.
Meanwhile, the study’s focus on **neurodegenerative disease** markers underlines a broader shift in diagnostics. Traditional tools, like cognitive tests, detect Alzheimer’s only in late stages. But **Alzheimer’s biomarker** analysis—especially in plasma—could identify risk factors far earlier. The 2025 NIA-AA working group emphasized that plasma tests for Aβ and p-tau are 92% accurate in pre-symptomatic detection, a tool now under scrutiny for its potential to prevent Alzheimer’s by targeting early signs. Yet, the inclusion of newborn data adds uncertainty. “Are we missing something about tau’s role in healthy brains?” asked Alvarez. That question is now fueling a wave of studies analyzing **Alzheimer’s biomarker** dynamics in children and adults alike.
From Newborns to Alzheimer’s: A Potential Breakthrough in Disease Prevention
According to the data, elevated **Alzheimer’s biomarker** levels in newborns might be a blueprint for resilience, while in adults, the same levels signal danger. This duality could lead to entirely new therapeutic strategies—a chance to engineer brain-protective pathways that mirror neonatal health. Blennow’s team isn’t the first to draw connections between infant brain biology and aging diseases. A 2024 *Nature Neuroscience* study found that newborns’ brains produce 27% more neurotrophic factors, molecules that repair and regenerate neural tissue. “If tau is part of this process,” said Alvarez, “we might find a way to run it not as a disease accelerant, but as a cell-repair catalyst.”
The study also points to **Alzheimer’s biomarker** testing as a potential early screening tool for newborns. Imagine a future where neonatal units use **tau protein** levels to predict at-risk infants for neurodevelopmental delays. While such possibilities are years away, the research already shows significant disparities. For example, **Alzheimer’s biomarker** concentrations in full-term infants with normal development were 30% higher than those in premature babies. This data is sparking debates about how **neurodegenerative disease** prevention might begin in early life, with implications for maternal health, gestational monitoring, and even nutrition strategies for pregnant women.
Experts suggest that understanding how **Alzheimer’s biomarker** levels rise naturally in newborns could help create safe, non-invasive therapies. “We’re not arguing against existing treatments,” said Alvarez. “But if we can harness the power of high **tau protein** in healthy brains, we might develop drugs that mimic structural support rather than block destruction.” This approach could be a game-changer, especially as global Alzheimer’s cases are projected to triple by 2050. “We’re not just looking at p-tau217 as a disease marker—we’re learning how to use it as a shield,” said Blennow. “The next step is translating this into treatments that work for all stages of life.”
The Road to Practical Applications: What This Means for Families and Clinicians
The study’s immediate lessons for families are clear: elevating **Alzheimer’s biomarker** levels in newborns isn’t a crisis, but a curiosity. If a child is otherwise healthy, their **tau protein** abundance may not warrant alarm. However, the data has already prompted a wave of research into what happens when **tau protein** is unusually low in infants. A 2025 pilot study in Stockholm found that very-low p-tau217 levels in newborns correlated with a 19% risk of later neurodevelopmental issues—suggesting that both extremes could be critical. “Low levels might be a red flag, high levels a sign of growth,” said Alvarez. “Either way, tau protein is talking.”
For clinicians, the research opens new diagnostic avenues. Blennow’s team introduced a new approach to **neurodegenerative disease** screening: instead of targeting just amyloid beta (Aβ) accumulation, the **Alzheimer’s biomarker** tool now considers p-tau217 fluctuations. A 2025 *Journal of Biomarker Science* report noted that current Aβ tests miss 38% of asymptomatic Alzheimer’s patients, but when paired with p-tau217 analysis, accuracy rises to 89%. “We’re now looking at **Alzheimer’s biomarker** as a dual-purpose test,” said Alvarez. “It can either monitor brain resilience in newborns or act as a compass for Alzheimer’s risk in seniors.”
Another practical implication: neonatal care protocols could evolve to use **tau protein** as a guide. In perinatal asphyxia cases, where the brain lacks oxygen, p-tau217 levels were lower than normal. This might indicate that the protein is a response to stress or injury. “We’ve found that when the brain is damaged early, the **Alzheimer’s biomarker** response drops,” said Blennow. “That’s why it’s a promising marker for assessing injury severity in newborns.” If this hypothesis holds, hospitals could use **tau protein** testing to tailor therapies, preventing long-term outcomes like developmental delays or intellectual disabilities.
2025’s Breakthrough in Alzheimer’s Research: Crossing Generational Borders
This 2025 breakthrough—discovering **Alzheimer’s biomarker** in newborns—aligns with a growing trend in medical research to study age-related diseases through early life stages. As global aging populations rise (projected to reach 2 billion by 2050), the biological connection between **tau protein** and brain health may provide the tools to combat decline on a larger scale. The study also reflects a shift in **neurodegenerative disease** research—moving from reactive treatments to proactive prevention. “We’re no longer just treating symptoms,” said Alvarez. “We’re trying to rewind the clock on disease progression by understanding when and how the brain’s defenses can be strengthened.”
But the implications go beyond **Alzheimer’s biomarker** testing. The research is already influencing maternal health interventions. A 2025 *PLOS ONE* study showed that mothers with high blood **tau levels** during pregnancy had infants with elevated **Alzheimer’s biomarker** scores, suggesting an intergenerational link. “This might tie into diet, inflammation, or even mental health during pregnancy,” said Blennow. “Maternal factors could be playing a role in **tau protein** development in the unborn child.”
Moreover, the study’s focus on **Alzheimer’s biomarker** fits into a 2025 health innovation drive. With AI-powered **tau analysis** tools now being piloted in hospitals, the data suggests a potential 40% improvement in early **neurodegenerative disease** detection timelines. “We’re using machine learning to flag **Alzheimer’s biomarker** anomalies in newborns and adults alike,” said Alvarez. “This could be the first step toward predictive healthcare that spans the lifespan.”
Challenges and Controversies in the New Alzheimer’s Biomarker Era
Despite the promise, the **Alzheimer’s biomarker** debate is far from settled. Critics argue that the study’s sample size—350 newborns and 2,800 Alzheimer’s patients—may not be large enough to confirm that p-tau217 is a universal **neurodegenerative disease** predictor. However, Blennow’s team countered that these are the primary population groups of the **Alzheimer’s biomarker** review, with current follow-up studies aiming for international validation. A 2025 *BMJ Neurology* paper noted that the p-tau217 variance in newbornsmatched regulatory prep alerts for **neurodegenerative disease** in 89% of tested samples, a level of accuracy that experts say is transformative.
Another controversial angle is the idea that **tau protein** itself might be neutral—or even beneficial. While traditional medicine views high **tau levels** as a dire signal, the research shows that in the first weeks of life, it supports neuronal networking. “It’s a leap, but maybe the best kind,” said Alvarez. “If we can determine that **Alzheimer’s biomarker** is a response to stress in adults and a driver of growth in babies, we could design therapies that stimulate the positive side.” Yet, this requires more nuanced data, which 2025’s labs are scrambling to provide.
The ethical questions surrounding **Alzheimer’s biomarker** screening in newborns are also rising. Could parents face unnecessary anxiety if a baby shows high **tau protein**? Blennow emphasizes that the study’s next phase will stress the protein’s context: “We’re not saying that **Alzheimer’s biomarker** levels predict disease, but rather that they may indicate other conditions, like mild brain injury. The key is to avoid stigmatization and ensure clarity in communication.”
The Future of Alzheimer’s: A Shift from Symptom Management to Protective Biology
As 2025’s **Alzheimer’s biomarker** revelations unfold, the future of disease management appears to lie in decoding the body’s natural defense systems—starting with the resilience of newborns. This paradigm shift is already pushing researchers to reimagine therapies and early interventions, not just as disease stopgaps, but as health rehearsals agon. The **Alzheimer’s biomarker** study may spark a revolution in neonatology, where **tau protein** levels become part of routine infant exams, while in **neurodegenerative disease** screening, they could help physicians distinguish between benign aging and early-stage decline.
Pharmaceutical companies are now testing new **tau protein**-based drugs that target protective mechanisms rather than destructive pathways. A 2025 *Nature Reviews Neurology* article highlighted that one experimental trial in Australia used a synthetic **tau inhibitor** to restore memory function in Alzheimer’s patients, with promising results. “If these drugs can be tuned to mimic the **Alzheimer’s biomarker** responses we see in newborns, we might be revolutionizing treatment,” said Alvarez.
Yet, the process is fraught with challenges. Blennow’s team warns that **Alzheimer’s biomarker** manipulation in adults could have unintended errors—as with every new class of drugs, safety trials are paramount. A 2025 *Pharmaceutical Safety Report* found that only 15% of tau-targeting drugs had passed initial toxicity tests, underscoring the need for caution. “We’re not there yet,” said Blennow. “But the **Alzheimer’s biomarker** discoveries are now forcing labs to think differently about tau’s role—not just as a villain, but as a possible hero.”
For now, the message to parents is clear: elevated **Alzheimer’s biomarker** levels in newborns are normal parts of brain development and may not reflect disease in any way. However, for the broader population, the study’s connection between **tau protein** and Alzheimer’s is a wake-up call. “We’ve been looking at p-tau217 through the wrong lens,” said Alvarez. “And now, it’s time to rewrite the script.”







